Last Updated
02/08/2023
General pediatricians, pediatric medical subspecialists and pediatric surgical specialists have a crucial role in providing frontline medical care to children and adolescents during the ongoing COVID-19 pandemic. As of February 2 , 2023, over 15.4 million children and adolescents have had laboratory-confirmed SARS-CoV-2 infection, representing 18.1% of all reported COVID-19 cases in the United States.1,2
COVID-19 vaccination is one of the most important and effective tools in protecting our children, families and communities. Vaccination with an authorized COVID-19 mRNA vaccine is strongly recommended for all eligible children 6 months of age and older, including booster vaccination of eligible children3; pediatric vaccination demonstrates effectiveness against acute COVID-19 and its complications, including multisystem inflammatory syndrome in children (MIS-C).3,4 COVID-19 vaccination is also recommended for household and close contacts of children, particularly in households with children who are not eligible for COVID-19 vaccination.
Changes in SARS-CoV-2 epidemiology, including the emergence of distinct SARS-CoV-2 variants and subvariants, have altered the landscape of options for SARS-CoV-2 directed therapies. These data are important in the management of high-risk patients with COVID-19.5.The US Food and Drug Administration (FDA) has approved and issued an Emergency Use Authorization (EUA) for early oral antiviral therapy for the treatment of mild to moderate COVID-19 in nonhospitalized, high-risk individuals in outpatient settings.6,7
Given dominant SARS-CoV-2 variants circulating in the United States, no monoclonal antibody (mAb) product is currently authorized for the treatment or pre-exposure prophylaxis of COVID-19.
- As of November 30, 2022, the mAb bebtelovimab is no longer authorized for treatment in any region in the United States because of lack of efficacy against circulating SARS-CoV-2 variants.10
- As of January 26, 2023, the mAb tixagevimab copackaged with cilgavimab (Evusheld) is no longer authorized for pre-exposure prophylaxis in the United States because of lack of efficacy against circulating SARS-CoV-2 variants.11 Retained product can be appropriately held for possible future use in the event that SARS-CoV-2 variants that are neutralized by Evusheld become more prevalent in the future.
No COVID-19 mAb product or antiviral medication is authorized for use as postexposure prophylaxis.
This interim guidance briefly summarizes currently available recommendations for the outpatient management of mild to moderate COVID-19 in children and adolescents at highest risk for disease progression and is also intended to help navigate management challenges and considerations, while acknowledging the following ongoing major limitations:
- Antiviral therapies have been authorized for individuals at highest risk for severe COVID-19 and disease progression, including those who may not be eligible for COVID-19 vaccination, have an underlying medical condition or are receiving therapies that are known to result in a poor antibody response to vaccination. Current evidence remains limited but is evolving regarding which underlying medical conditions in children definitively associate with higher risk for severe COVID-19 or disease progression.
- There continues to be a paucity of pediatric-specific data regarding the safety, efficacy and pharmacokinetics of oral antiviral medications across all pediatric age groups.
The National Institutes of Health (NIH) COVID-19 Treatment Guideline Panel provides a framework for assessing risk of progression to severe COVID-19 based on underlying medical conditions, vaccination status, illness severity and age.7 Given lack of pediatric clinical trial data, the recommendations for the therapeutic management of COVID-19 in nonhospitalized children are based primarily on safety and efficacy data from clinical trials in adults, and thus, the quality of the evidence is based on expert opinion. The NIH Panel’s current treatment recommendations for mild to moderate COVID-19 in symptomatic nonhospitalized children and adolescents at highest risk for progression to severe COVID-19 may include13:
- Paxlovid (if within 5 days of symptom onset)
or - remdesivir (if within 7 days of symptom onset)
Which children and adolescents are considered “high risk” for COVID-19 disease severity and complications and may qualify for outpatient COVID-19 directed treatments?
Strong or consistent criteria associated with high risk for severe COVID-19 in children and adolescents include7,14:
- Obesity, defined as BMI ≥95th percentile for age and sex based on CDC growth charts, especially severe obesity, defined as BMI ≥120% of 95th percentile for age)
- Immunosuppressive disease or receipt of immunosuppressive therapies resulting in moderate or severe immunocompromisec
- Neurodevelopmental disorders (eg, cerebral palsy, trisomy 21) that result in impaired airway clearance
- Medical complexity, including medical-related technological dependence that is not related to COVID-19 (eg, tracheostomy, positive pressure ventilation, gastrostomy)
- Severe congenital or acquired heart disease
- Severe chronic lung disease (eg, interstitial lung disease); severe asthma or other chronic respiratory disease that requires daily medication for control
- Multiple moderate to severe chronic diseases
- Pregnancy
Conditions with moderate or inconsistent association with progression to severe COVID-19 in children:
- Sickle cell disease
- Diabetes (poorly controlled)
- Chronic kidney disease
- Chronic liver disease (eg, cirrhosis, autoimmune hepatitis)
- Nonsevere cardiac, neurologic, or metabolic disease
- Age <1 year
- The majority of infants have mild, uncomplicated SARS-CoV-2 infection. Published case report data suggest that young infants, particularly those <90 days of age, are hospitalized more frequently; however, the indication for hospitalization may be confounded by need for evaluation of fever in these infants who otherwise have mild COVID-19 symptoms and good outcomes.15-18
- Data evaluating risk factors for severe COVID-19 in young infants, in whom severity is defined by requiring admission to the intensive care unit or mechanical ventilation, have identified prematurity (gestational age <37 weeks) as a possible risk factor for severe COVID-19.19-21
Conditions with weak or unknown association with progression to severe COVID-19:
- Mild asthma
- Overweight
- Diabetes (well controlled)
SARS-CoV-2 therapies are not routinely indicated for children/adolescents with COVID-19 at low risk for progression or hospitalization.
Providers will need to continue to evaluate multiple factors when assessing potential risks and benefits of therapy and selecting possible treatment options for an individual patient, including and not limited to the clinical efficacy of agents, availability of the treatment in their geographic area, feasibility of administering the medication, local and regional SARS-CoV-2 epidemiology including variant and subvariant types, host-specific factors including concurrent medical conditions and possibility of drug-drug interactions and available safety data for these medications in children. Providers should refer to the risk assessment framework to identify children at highest risk for severe COVID-19 and who may benefit from receiving COVID-19-directed treatment.7
What therapies are available to treat and prevent the progression of mild to moderate COVID-19 in high-risk children and adolescents?
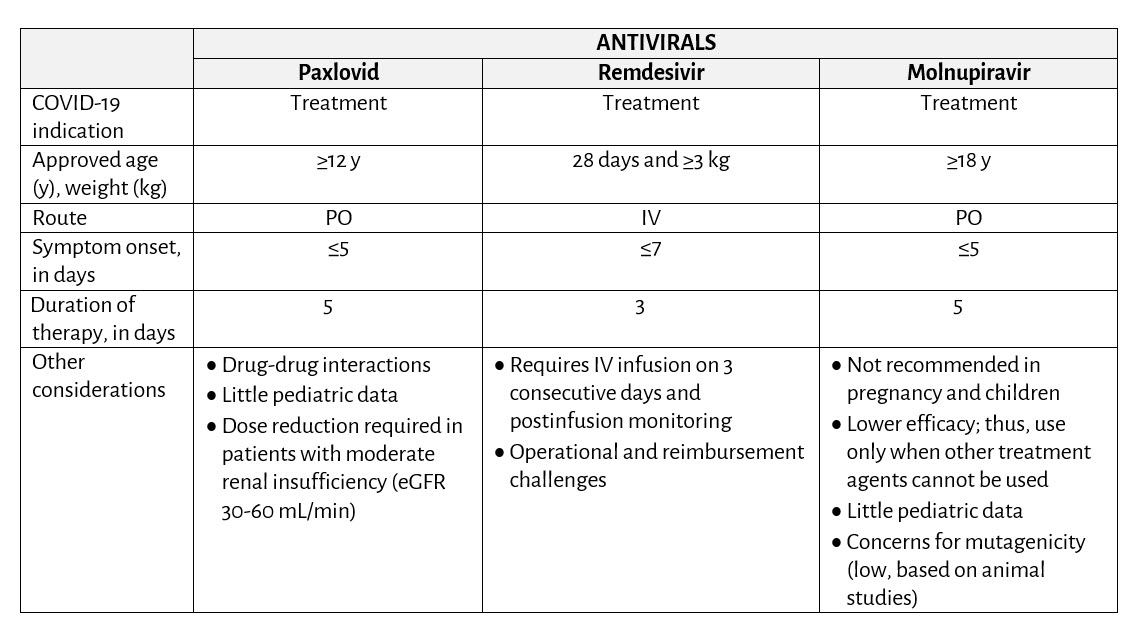
- The early use of intravenous (IV) remdesivir22 and oral antiviral medications (Paxlovid51 and molnupiravir50) have been evaluated in controlled clinical trials among nonhospitalized, high-risk individuals with mild to moderate COVID-19 and found to improve COVID-19 outcomes including disease progression, hospitalization and death. Current NIH recommendations favor the use of Paxlovid or remdesivir for mild to moderate COVID-19 in high-risk outpatients7; alternative therapies, such as molnupiravir, may be considered in individuals ≥ 18 years of age, if the preferred options are not available or clinically cannot be used. Table 1 summarizes the currently authorized options.
- IV remdesivir: Remdesivir is the only drug approved by the FDA for the treatment of COVID-19 in children 28 days and older and weighing at least 3 kg who are hospitalized or not hospitalized and have mild to moderate COVID-19 and are at high risk for disease progression. In a randomized, double-blind, placebo-controlled trial of nonhospitalized patients ≥12 years of age testing positive for SARS-CoV-2 and having at least one high-risk factor for COVID-19 disease progression, receipt of early remdesivir (given within 7 days of symptom onset) once daily for 3 days resulted in an 87% lower risk of hospitalization or death among 279 subjects receiving remdesivir than among 283 subjects receiving placebo by day 28.22 A phase 2/3 single-arm, open-label study that included 53 children at least 28 days of age weighing at least 3 kg and receiving remdesivir for up to 10 days demonstrated that the safety and pharmacokinetics of remdesivir in young children were similar to those in adults.24
- Logistical considerations may limit the use of IV remdesivir in the outpatient setting.
- Remdesivir IV for mild to moderate COVID-19 is approved for use in:
- adults and children 28 days of age and older and weighing at least 3 kg, and
- with laboratory-confirmed SARS-CoV-2, and
- who are within 7 days of symptom onset, and
- who are at high risk for progression to severe COVID-19
- The remdesivir IV dosage depends on age and weight as follows:
- 3 kg to 40 kg: remdesivir 5 mg/kg on day 1 (loading dose), followed by 2.5 mg/kg once daily on day 2 and 3
- ≥12 years and ≥40 kg: remdesivir 200 mg on day 1 (loading dose), followed by 100 mg, IV, daily on days 2 and 3
- The dose is given once daily on 3 consecutive days; the IV infusion is administered over 30 to 120 minutes.
- Adverse events were reported infrequently compared with placebo (0.7% vs 5.3%) and included nausea, headache and cough. Allergic reactions and increased liver enzymes are also possible side effects. Patients should be monitored during and for at least 1 hour after the dose for possible infusion-related reactions.
- Refer to the prescribing information for additional details.
- Oral antivirals (Table 2): There are no available data regarding the safety, efficacy or pharmacokinetics of SARS-CoV-2 oral antivirals in children <12 years of age. Additional information will be added to this interim guidance as more pediatric data become available.
- Paxlovid25
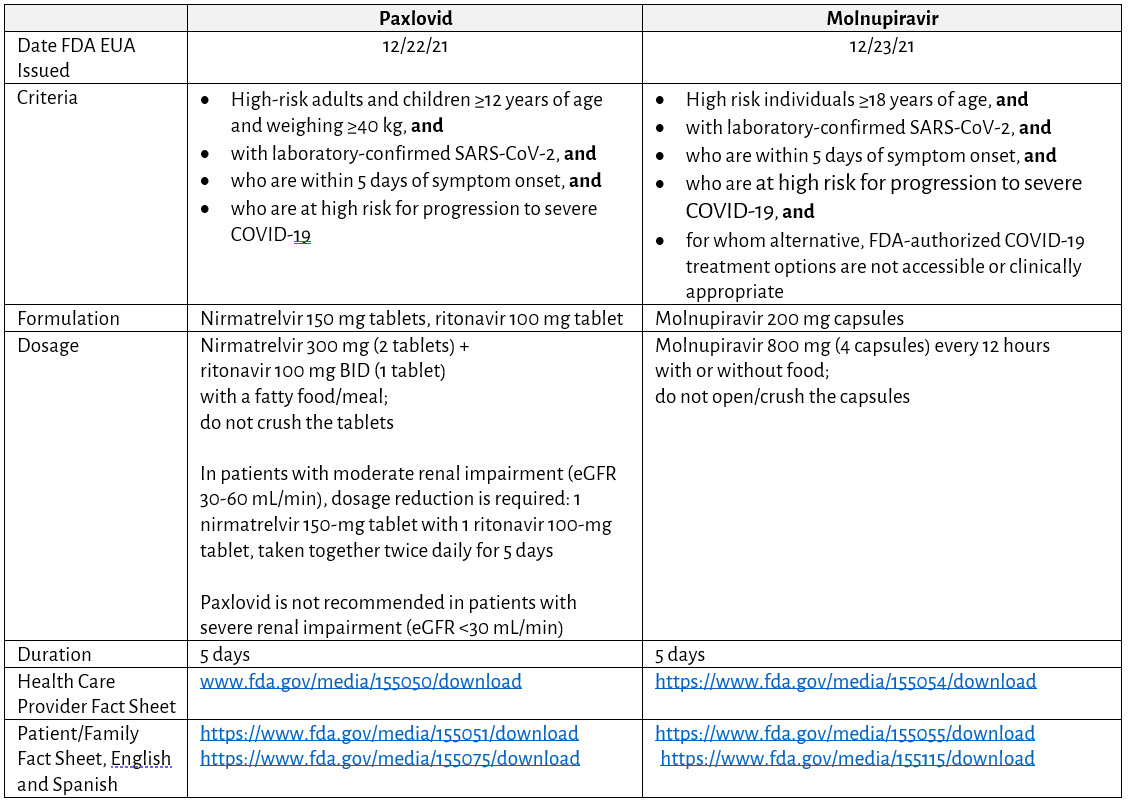
- The FDA issued an EUA for the use of Paxlovid for the treatment of mild to moderate COVID-19 on the basis of results of a phase 2/3 randomized, double-blind, placebo-controlled trial in high-risk individuals ≥18 years of age. Receipt of Paxlovid resulted in 88% relative risk reduction in COVID-19-related hospitalizations or death from any cause compared with individuals who received placebo through day 28; no children were included in this trial. Of note, 98% of SARS-CoV-2 variants in this trial were Delta; preliminary cell culture-based data suggest that nirmatrelvir retains antiviral activity against the SARS-CoV-2 Omicron variant.28-30 More recent data (April-August 2022) indicate that individuals ≥18 years of age prescribed Paxlovid within 5 days of COVID-19 diagnosis had a 51% lower hospitalization rate in the ensuing 30 days than individuals not been prescribed Paxlovid; irrespective of prior COVID-19 infection or vaccination status. A phase 2 and 3 clinical trial assessing the safety and efficacy of Paxlovid treatment in children 6 to 17 years of age with COVID-19 is underway.
- Paxlovid is copackaged as a combination of nirmatrelvir (a SARS-CoV-2 protease inhibitor, dispensed as two 150-mg tablets) and 100 mg of ritonavir (has no SARS-CoV-2 antiviral activity, but as a CYP3A inhibitor, increases nirmatrelvir blood concentrations). There are no pharmacokinetic/pharmacodynamic data in children; the authorized dose is expected to result in comparable serum drug concentrations in patients ≥12 years of age and weighing ≥40 kg.28
- The EUA authorizes the use of Paxlovid in:
- adults and children ≥12 years of age and weighing ≥40 kg, and
- are within 5 days of symptom onset, and
- who are at high risk for progression to severe COVID-19, and\
not hospitalized for COVID-19
On February 1, 2023, the FDA updated the Paxlovid EUA to no longer require positive results of direct SARS-CoV-2 viral testing. However, some experts continue to recommend that SARS-CoV-2 testing be performed in symptomatic individuals to help diagnose COVID-19, distinguish it from other respiratory viruses co-circulating in communities, and help determine appropriate antiviral therapies.
- If providers consider the benefit of treatment outweighs the potential risk in an individual, eligible patient regardless of COVID-19 vaccination status, then Paxlovid should be initiated within 5 days of symptom onset; the 3 tablets should be taken together, preferably with a fatty meal, and swallowed whole, twice daily, for a maximum of 5 days. Patients who are prescribed Paxlovid as outpatients but worsen and require hospitalization for any reason (including worsening of COVID-19 symptoms) may complete the 5-day course under the EUA and per provider discretion.
- The most frequent adverse events reported in clinical trials with Paxlovid include dysgeusia (metallic taste, 6% vs <1%), diarrhea, hypertension and myalgias. Refer to the Fact Sheets for Healthcare Providers32 and for patients/caregivers33 for additional details. In addition, providers should be aware of the possibility of recurrence of milder symptoms and/or a new positive viral test (after having tested negative) that has been reported to occur 2 to 8 days after initial recovery and despite antiviral therapy, referred to as “COVID-19 rebound.”
- Paxlovid should not be used in individuals with severe hepatic (eg, Child-Pugh Class C) or renal impairment (creatinine clearance, CrCl <30 mL/min). Dosage adjustments are required for patients with moderate renal impairment (eg, CrCl 30-60 mL/min), with doses available in a different packaging configuration (Paxlovid 150 mg; 100 mg Dose Pack). In addition, clinicians should thoroughly review the medications the patient is taking; there is a potential for severe drug-drug interactions if given concurrently with other medications that rely on or induce CYP3A enzymes for metabolism and clearance. Ritonavir may reduce the efficacy of combined hormonal contraceptives; patients should be counseled on an effective alternative method of contraception.
- Molnupiravir
- The FDA issued an EUA for the use of molnupiravir for the treatment of mild to moderate COVID in high-risk, nonhospitalized adults for whom alternative COVID-19 treatment options are not clinically available or appropriate. This EUA was based on results of a phase 3 randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of molnupiravir given within 5 days of symptom onset; molnupiravir led to a 30% reduction in the risk of all-cause hospitalization and death compared with placebo in nonhospitalized and unvaccinated, high-risk individuals ≥18 years of age through day 29. Children were not eligible to participate in the study because of concerns about effects on bone and cartilage growth.
- Molnupiravir is the oral ribonucleoside prodrug, available in 200-mg capsules; dosage is 800 mg (4 capsules) twice a day for 5 days.
- The EUA authorizes the use of molnupiravir in:
- Individuals ≥18 years of age, and
- who are within 5 days of symptom onset, and
- who are at high risk for progression to severe COVID-19, and
- for whom other, FDA-authorized COVID-19 treatment options are not readily accessible, available, or clinically appropriate12,38
Molnupiravir is not authorized for initiation of treatment in patients requiring hospitalization for COVID-19.
On February 1, 2023, the FDA updated the molnupiravir EUA to no longer require positive results of direct SARS-CoV-2 viral testing. However, some experts continue to recommend that SARS-CoV-2 testing be performed in symptomatic individuals to help diagnose COVID-19, distinguish it from other respiratory viruses co-circulating in communities, and help determine appropriate antiviral therapies. - Molnupiravir has lower efficacy than the preferred treatment options in adults with COVID-19. If providers consider the benefit of treatment outweighs the potential risk in an individual, then molnupiravir should be initiated as soon as possible and within 5 days of symptom onset. Patients who are prescribed molnupiravir as outpatients but worsen and require hospitalization for any reason (including worsening of COVID-19 symptoms) may complete the 5-day course under the EUA and per provider discretion.
- Adverse reactions reported to occur in ≥1% of clinical trial participants include diarrhea, nausea, and dizziness. No dosage adjustments are needed in patients with renal or hepatic impairment of any degree. Molnupiravir is not recommended for use in pregnancy given potential of fetal harm on the basis of animal data. Individuals of reproductive potential should be advised of the potential risk to the fetus and counseled regarding use of a reliable method of contraception during the treatment course and for 4 days after the last dose and be aware of the pregnancy surveillance program. Pregnant individuals should be encouraged to participate in the pregnancy surveillance program by calling 1-877-4231. In addition, molnupiravir should not be used if breastfeeding, including during the 5-day treatment and for 4 days following the last dose.
- Current NIH guidelines recommend the use of molnupiravir in eligible patients only when Paxlovid or remdesivir cannot be used. Refer to the Fact Sheets for health care providers and patients/caregivers and patients/families for additional details.
- Paxlovid25
Providers should review the antiviral fact sheets with eligible patients, provide patients an electronic or hard copy of the fact sheet, and document review, receipt, and counseling recommended within the fact sheets.33,39
The oral SARS-CoV-2 antivirals are not authorized to be initiated in children/adolescents who are hospitalized for COVID-19 or as pre- or postexposure prophylaxis. Providers should follow FDA EUA requirements and report side effects and adverse events related to antiviral therapies within 7 calendar days (1-800-FDA-1088).
What are additional considerations for children receiving COVID-19 directed therapies?
- Despite receiving SARS-CoV-2 therapies, clinical worsening of COVID-19 has been reported and may include fever, hypoxia or increased respiratory distress, dysrhythmias and altered mental status. Pediatricians, pediatric medical subspecialists and pediatric surgical specialists should advise parents/caregivers on how to monitor for clinical worsening, occurring most frequently in the first 7 to 10 days after symptom onset and provide further instructions on when to seek emergency medical attention.
- Receipt of COVID-19 directed therapies does not preclude the need to continue to follow recommended preventive measures, including wearing an appropriately fitted mask in children and adolescents ≥2 years of age, physical distancing and performing hand hygiene.
- Eligible children ≥6 months of age and their household contacts should receive COVID-19 vaccine when available to them. After SARS-CoV-2 infection, COVID-19 vaccination can be provided once symptoms resolve and the recommended isolation period has ended. Individuals who have received COVID-19 mAb for preexposure prophylaxis can receive their COVID-19 vaccine at any time.
What strategies may be considered in communities with resource constraints or limited access to SARS-CoV-2 therapies?
- The COVID-19 pandemic has further highlighted ongoing health care disparities.50 The American Academy of Pediatrics (AAP) strongly supports the equitable distribution and availability of therapeutic medications and vaccinations to all eligible children and adolescents.
- There may be varying access to SARS-CoV-2 antivirals geographically, requiring additional triage for patients at highest risk for COVID-19. State and local health departments will allocate therapies to health care facilities. Considerations when assessing COVID-19 risk and prioritizing SARS-CoV-2 therapies for an individual patient should take into account host, situational and ethical factors, including:
- Prioritizing the treatment of SARS-CoV-2 infection in patients at highest risk for COVID-19 complications and hospitalizations.12
- When outpatient therapies are extremely limited, prioritizing among the most severely immunocompromised individuals may be prudent for treatment, followed by severely immunocompromised with additional COVID-19 risk factors.
- COVID-19 vaccination status: Individuals who are unvaccinated or incompletely vaccinated against COVID-19 or fully vaccinated individuals ≥2 weeks after completing their COVID-19 mRNA primary vaccine series but not expected to mount an adequate vaccine immune response are at higher risk for hospitalization than fully vaccinated, nonimmunocompromised individuals.52-54
What medications should not be used to treat or prevent the progression of COVID-19 in children and adolescents?
- There is NO conclusive evidence to support the efficacy and safety of the following medications for routine use in the treatment or prevention of COVID-19 in children and adolescents. It is strongly recommended that these unproven interventions not be prescribed and parents be counseled against their use. In addition to showing no efficacy against COVID-19, inappropriate use of these antimicrobials cause significant harm.55,56 The following are NOT recommended to be prescribed for COVID-19:
- Azithromycin: Results of randomized trials in ambulatory subjects conclude that azithromycin did not result in more or faster COVID-19 symptom improvement compared with placebo and had no meaningful benefit in preventing COVID-19 hospitalizations.57,58
- Ivermectin59: Inappropriate use of this antiparasitic in patients with COVID-19 has led to increased reports to poison control centers of severe illness and has prompted a CDC Health Advisory.60
- Hydroxychloroquine/chloroquine: Moderate-quality evidence suggests that these agents lack efficacy in reducing short-term mortality or need for hospitalization in patients with COVID-1961; in addition, serious cardiac events, including QTc prolongation, have been reported.
- Systemic corticosteroids are NOT recommended for treating patients with mild to moderate COVID-19 who do not require supplemental oxygen for their SARS-CoV-2 infection, given lack of proven benefit and possible harm. Recent updates to the COVID-19 treatment guidelines recommend against the use of dexamethasone or other systemic glucocorticosteroids for this indication, in this population.38
Additional Information
There is much misinformation on the internet/social media. Pediatricians, pediatric medical subspecialists and pediatric surgical specialists are encouraged to refer patients and families to reputable, up-to-date COVID-19 resources:
- NIH COVID-19 treatment guidelines
- IDSA Guidelines on the Treatment and Management of Patients with COVID-19
- AAP Red Book chapter: Coronaviruses, Including SARS-CoV-2 and MERS-CoV
- The Healthy Children website: www.healthychildren.org
Table 1. Summary of Current Potential SARS-CoV-2 Antivirals Based on Indication and EUA Age/Weight Inclusion Criteria in Children and Adolescents at High Risk for Progressing to Severe COVID-19
IM, intramuscular; IV, intravenous; PO, per os; n/a not applicable.
Table 2. Summary of SARS-CoV-2 Oral Antivirals Currently Authorized for Use in Mild to Moderate COVID-19
Interim Guidance Disclaimer: The COVID-19 clinical interim guidance provided here has been updated based on current evidence and information available at the time of publishing.
Last Updated
02/08/2023
Source
American Academy of Pediatrics