The presence of recommendations is what separates clinical guidelines from other types of medical literature (eg, original research, reviews, commentaries). Recommendations tell health care professionals what to do and when. Well-articulated recommendations can be translated easily into IF-THEN statements that facilitate implementation and numerators and denominators that define quality measures. It is important to note that recommendations—not guidelines—represent the units of implementation—that is, it is possible to implement one or a few recommendations from one or more guidelines to achieve a quality improvement goal.
Structure and Content of CPG Recommendations
To promote clarity, it is useful for clinical recommendations to be explicit about:
- WHEN, ie, under precisely what circumstances,
- WHO is the guideline’s intended audience,
- OUGHT, with what level of obligation,
- To do exactly WHAT to WHOM.
Such a key action statement should be followed by text amplifying:
- HOW the recommendation is to be performed, and
- WHY the guideline authors made the recommendation—that is, what its evidence base is.
Key Action Statements (KASs) should be formatted to be immediately recognizable as such, for example using boldface fonts. For example: If an infant under the age of 2 is diagnosed with X, then pediatricians should prescribe drug Y at a dose of 100 mg/kg for 5 days.
Ambiguous, Vague, and Underspecified Language (AVUL)
Ambiguous recommendations are interpretable in more than one discrete way. True ambiguity is uncommon and is most often the result of mixing logical operators (ANDs and ORs) in the same statement or using abbreviations with multiple meanings. More commonly, vague and underspecified recommendations are developed that lack crisp thresholds or specificity.
Sometimes authors deliberately create vague and underspecified recommendations. This occurs when:
- there is insufficient evidence to be explicit;
- teams are unable to reach consensus;
- there are legal concerns (eg, fear of setting a standard of care); or
- there are economic reasons for vagueness.
If authors insist on creating vague statements (which will necessarily be more difficult to implement), they should be clear about the reason for the vague recommendation. Such explanations help users to understand how to interpret and apply the underspecified advice. Because recommendations define what practitioners should DO, it is important to use active voice (passive voice masks the responsible actor) and transitive verbs—those that act upon an object. Careful verb selection promotes clear articulation of intent.
Levels of Obligation
Recommendations impose obligation. In certain circumstances, for example, when evidence quality is high, and benefits far outweigh potential harms, high levels of obligation may be imposed. Alternatively, when evidence quality is low and/or benefits are closely balanced against potential harms, recommendations are more informative and do connote lower levels of obligation. It is important that recommendations convey the appropriate level of obligation intended by the guideline authors.
Statements of Fact
Recommendation writers regularly label statements of fact as recommendations when there is no implied action. For example:
- Recommendation 3. Pneumatic otoscopy is the most accurate test for otitis media with effusion.
In this example, no action is called for. Rewriting this information into a directive statement creates an action statement:
- Recommendation 3. Clinicians should use pneumatic otoscopy as the primary diagnostic method for otitis media with effusion.
The Use of "Consider"
It is tempting to use the term “consider” to water down a recommendation when there is little evidence to support it or benefits are closely balanced against harms. Unfortunately, it is difficult or impossible to measure whether an action has been considered. One can only measure whether it has been performed.
Evidence Quality and Recommendation Strength
Many guideline authors conflate 2 related concepts that should remain distinct: quality of evidence and recommendation strength. Failing to separate these concepts results in confusion for users attempting to implement the recommendation.
Evidence quality refers to “the extent to which all aspects of a study’s design and conduct can be shown to protect against bias and inferential error.”
Recommendation strength indicates the authors’ expectation of the level of adherence to a recommendation. Clinicians should follow strong recommendations unless a clear and compelling rationale for acting in a contrary manner is present.
Role of Values
The process of formulating a guideline recommendation requires that the developers judge whether the anticipated benefits of following the recommendation will outweigh risks, harms, and costs of doing so, or whether harms will outweigh benefits. In many cases, there will be an apparent equilibrium in which positive outcomes are expected but at significant risk. Guideline authors apply their personal values to the evidence that is identified regarding each recommendation. Differences in the values applied help to explain conflicting recommendations that come from highly reputable sources—for example, breast cancer screening recommendations from the American College of Radiology and the American Cancer Society conflict with those from the US Preventive Services Task Force. Each organization reviewed the same evidence but came to different conclusions. It is important for authors to be transparent about the values they apply in creating recommendations. An effective mechanism for enforcing transparency is for authors to enumerate the anticipated benefits and harms and to make a clear statement that they believe that one or the other predominates or benefits and harms are balanced.
Key Action Statement Profiles
There are multiple guideline tools available to assist organizations with developing and formatting key clinical recommendations. There is not one specific software program endorsed by the AAP. Each guideline recommendation should be accompanied by a Key Action Statement Profile. The KAS profile summarizes the facts and judgments that support and define the recommendation. Each profile should include:
- Aggregate Evidence Quality supporting the recommendation
- Recommendation Strength
- Anticipated Benefits
- Anticipated Risks, Harms, and Costs
- Value Statement, for example, “The CPG Subcommittee believes that the benefits listed above outweigh the risks, harms, and costs described.”
- Deliberate Vagueness (if applicable)
- Specific Exclusions (if applicable)
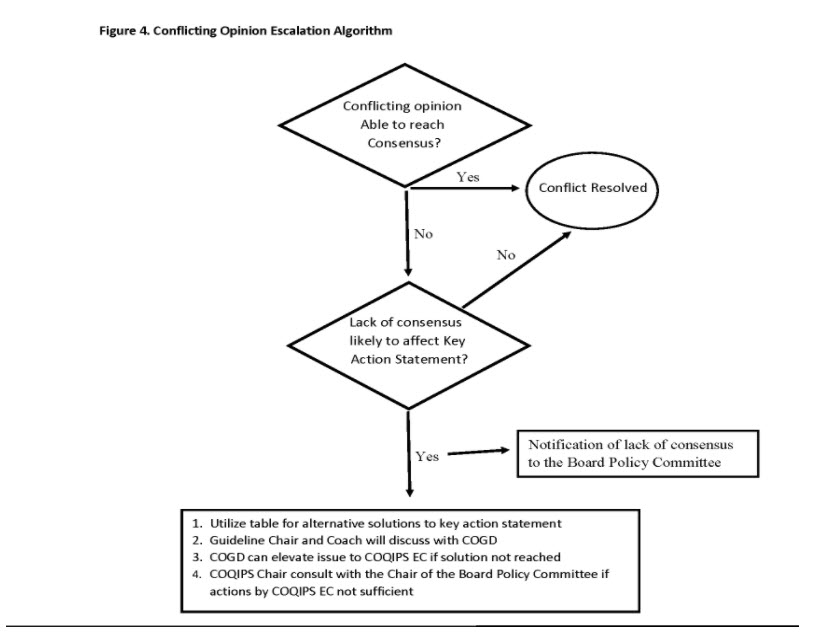
Mitigation of Conflict and Dissenting Opinions
During the guideline development process, conflicts involving differing opinions between committee members or other stakeholders can arise leading to disagreements regarding the guideline recommendations or key action statements. These conflicts can arise from a variety of reasons including diverse expertise and experience between stakeholders, differing interpretation of the evidence, and a variable level of risk tolerance, amongst others.
Differing opinion and a certain level of conflict is not only expected, but essential during guideline development. However, when these differences become irreconcilable and become a barrier to achieving consensus, the guideline process could be significantly delayed or disrupted.
One of the primary roles of the guideline Chair is to proactively manage conflict. The first and most important step to conflict resolution is prevention. The Chair should establish ground rules for conduct and decision-making at the first meeting. During meetings, the Chair will reinforce these ground rules and continually re-enforce them over the course of guideline development. In addition to the Chair, a Guideline Coach will be available to assist with the resolution of dissenting or conflicting opinions of guideline subcommittee members.
As the most controversy typically arises during the development of key action statements, it is important to adhere to the AAP process for developing these statements. Committees are to be transparent about all of the factors that are considered (e.g., potential harm, unintended consequences, patient preferences) and views of all of the stakeholders (families, providers, subspecialists, payors).
To better support the Chair during the guideline development process, the guideline Chair and Coach will report on the status of the guideline quarterly to the COGD. Part of this report will include whether the Chair anticipates or is already grappling with challenges with coming to consensus on key action statements. At the call, strategies for resolving these conflicts will be identified.
If the conflicts are deemed so severe that the guideline development is at risk, further escalation is needed. COGD will involve the COQIPS Executive Committee and/or COQIPS Chair. Further escalation steps will involve the Board Policy Committee or the AAP Board of Directors.
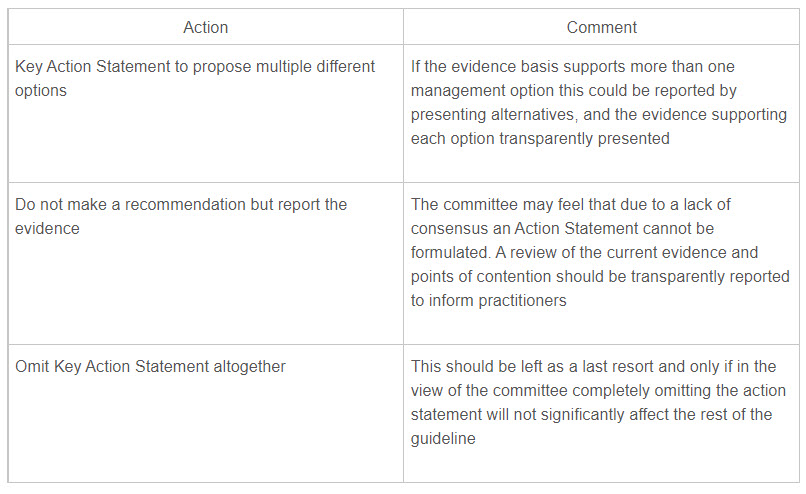
The table below presents possible solutions for conflicts (Table 2).
Table 2. Alternative solutions when consensus on action statements cannot be reached
Last Updated
05/10/2024
Source
American Academy of Pediatrics