The AAP Influenza Policy for the prevention and control of influenza is updated every year by both the American Academy of Pediatrics (AAP) and the Centers for Disease Control and Prevention (CDC) prior to the start of the influenza season. The AAP statement is pediatric-focused, while the CDC statement provides guidance for people of all ages. Through their advisory committees, the AAP Committee on Infectious Diseases (COID) and the Advisory Committee on Immunization Practices (ACIP) of the CDC, have reviewed and carefully considered all influenza vaccine efficacy data available to date, and recommend both inactivated influenza vaccine (IIV) and live attenuated influenza vaccine (LAIV) as options for influenza vaccination in children for the 2020-2021 influenza season, with no preference.
This algorithm from the AAP influenza policy statement describes the number of 2020-21 seasonal influenza doses for children based on age and prior vaccination history.
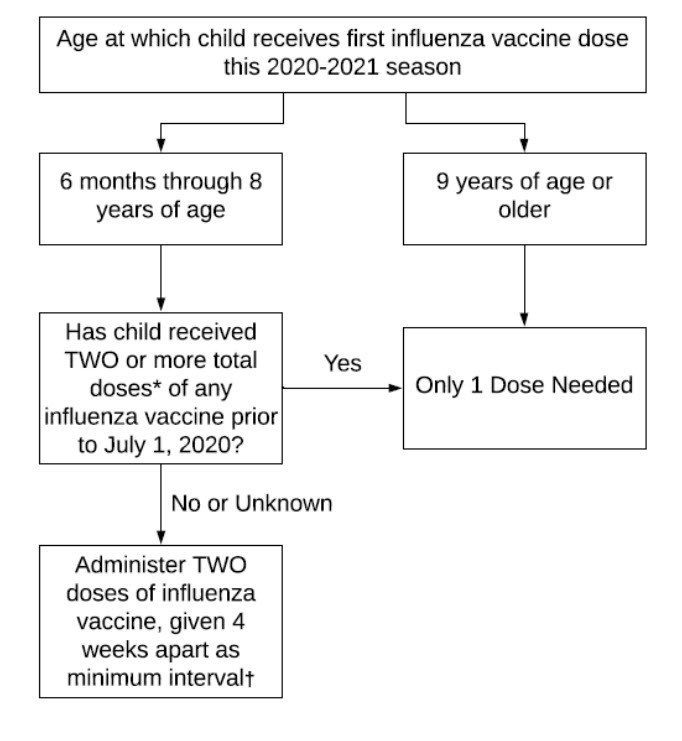
* The 2 doses need not have been received during the same season or consecutive seasons.
† Administer 2 doses based on age at receipt of the first dose of influenza vaccine during the season. Children who receive the first dose prior to their ninth birthday should receive 2 doses, even if they turn 9 years old during the same season.
Last Updated
07/26/2021
Source
American Academy of Pediatrics