Authors found that pediatric clinical trials lacked participant diversity that was representative of the U.S. population in research presented at the 2021 American Academy of Pediatrics National Conference & Exhibition
ITASCA, IL – When conducting pediatric clinical trials, the scientific process is best served when participants come from diverse racial and ethnic backgrounds, so that conclusions are more likely to be generalizable to a broad population. Yet, according to an analysis of articles on U.S. pediatric clinical trials, Black children were enrolled at a proportionally higher rate than their representation in the U.S. population, while other populations were under-represented.
Researchers will present the study abstract, “Race and Ethnicity in Published Pediatric Clinical Trial Enrollment in the United States, 2011-2020,” during the virtual American Academy of Pediatrics 2021 National Conference & Exhibition.
“Our study identifies key areas in which we, as a pediatric research community, can improve enrollment in clinical trials to be more equitable for all groups,” said study author Chris A. Rees, MD, MPH, pediatric emergency medicine physician at Children’s Healthcare of Atlanta and assistant professor of pediatrics and emergency medicine at Emory University, who performed this study along with researchers from Boston Children’s Hospital.
“Results from clinical trials that lack American Indian, Alaska Native, Asian, and Native American-Pacific Islander participants may not be generalizable to all populations that may benefit from trial results,” he said.
The cross-sectional study reviewed 612 articles published in five leading general pediatric and five leading general medical journals from January 1, 2011-December 31, 2020. Researchers determined the reporting of participant race and ethnicity in published clinical trial results, comparing the number of children enrolled with U.S. Census populations of pediatric racial and ethnic groups.
Black children were enrolled in higher proportions than their representation in the United States. Hispanic and Latino children were enrolled commensurate with their population, and American Indian, Alaska Native, Asian, and Native American-Pacific Islander children were enrolled significantly less relative to their population, the authors found. White children were enrolled less than expected based on their representation in the U.S. population, but made up 46% of participants in trials reporting race or ethnicity. More research is needed to determine the cause of these differences, but authors hypothesize they may be due to the locations where pediatric clinical trials are performed, the types of diseases and conditions that are studied, or may represent disparities in the trial enrollment process.
Researchers also observed that a substantial number of trials did not report participant race or ethnicity at all.
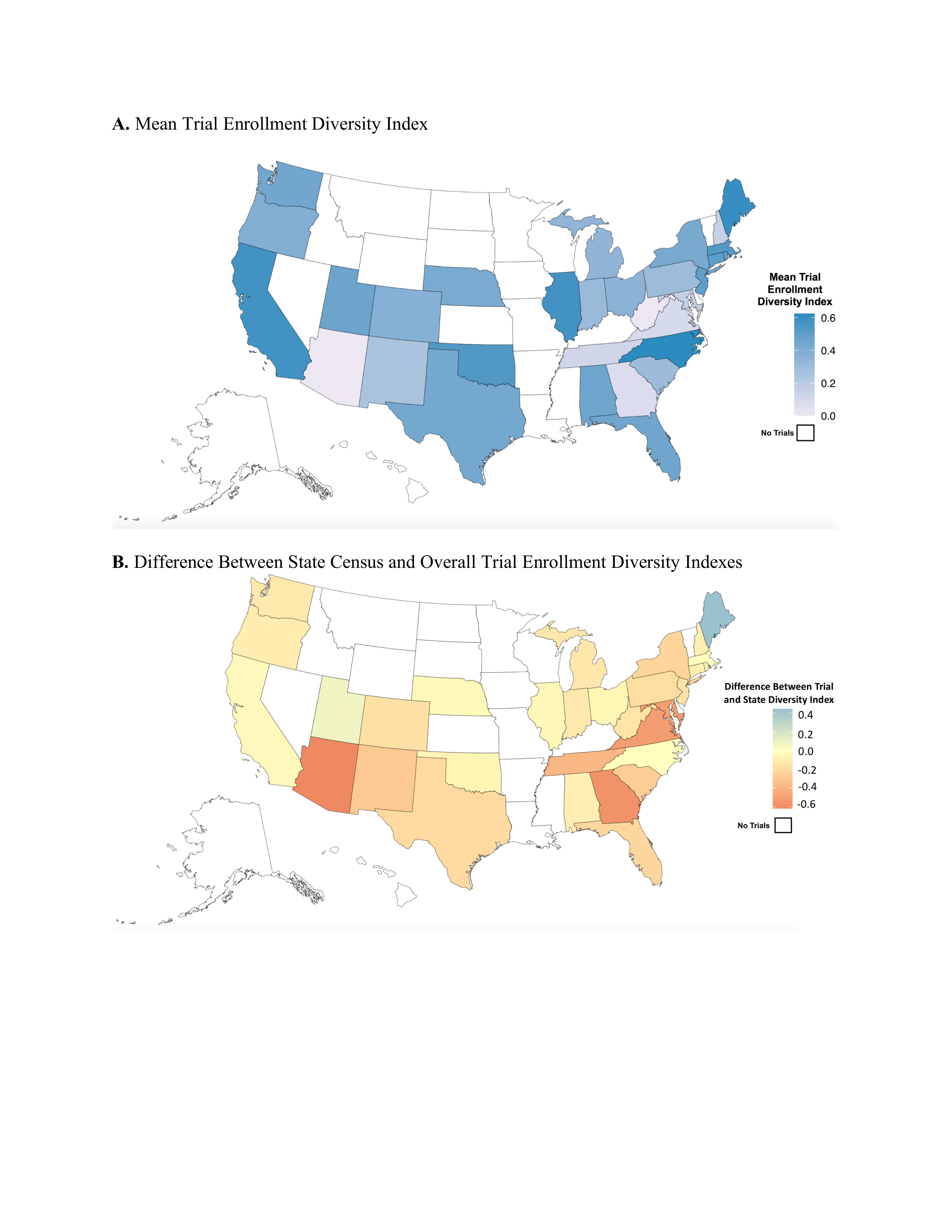
Pediatric clinical trials conducted exclusively in Arizona, Tennessee, Georgia, West Virginia, and New Hampshire had the lowest mean diversity indices and those conducted in California, Maine, Illinois, and North Carolina had the highest diversity indices, indicating that the representativeness of clinical trials may differ by state.
The research is supported in part by the National Institutes of Health.
Dr. Rees will present the study abstract as a poster presentation.
To request an interview, journalists may email Jennifer Burkhardt with Children’s Healthcare of Atlanta.
Please note: only the abstract is being presented at the meeting. In some cases, the researcher may have more data available to share with media or may be preparing a longer article for submission to a journal.
# # #
The American Academy of Pediatrics is an organization of 67,000 primary care pediatricians, pediatric medical subspecialists and pediatric surgical specialists dedicated to the health, safety and well-being of infants, children, adolescents and young adults. For more information, visit www.aap.org.
ABSTRACT
Abstract Title: Race and Ethnicity in Published Pediatric Clinical Trial Enrollment in the United States, 2011-2020
Chris Rees
Atlanta, GA, United States
Monday, October 11, 2021: 3:31 PM –
Equitable representation of participants who are Black, Indigenous, and People of Color in clinical trials enhances inclusivity in the scientific process and generalizability of results.
We conducted a cross-sectional study of published articles reporting pediatric clinical trials conducted in the U.S. and published in five leading general pediatric and five leading general medical journals from January 1, 2011-December 31, 2020. We determined the reporting of participant race and ethnicity in published clinical trial results. We compared the number of children enrolled versus U.S. Census populations of pediatric racial and ethnic groups. We calculated the mean diversity index of all trials by year using linear regression. We calculated mean diversity indices of single-state trials and compared the calculated trial diversity indices to those of the respective state.
There were 612 articles reporting pediatric trials during the study period with 565,618 total participants (median per trial 200 [interquartile range 90-571]). Of the 612 articles, 486 (79.4%) reported participant race and 338 (63.4%) reported participant ethnicity. Reporting of participant race increased by 7.9% (95% confidence interval [CI] 0.2-16.3) and of ethnicity increased by 11.4% (95% CI 4.8-18.4) from 2011-2020 (Figure 1). However, the mean annual diversity index of pediatric clinical trials did not change (0.002 percentage points/year, 95% CI -0.007-0.010). Among articles reporting race and ethnicity, the method of assignment was not reported in 51.1% and 71.6% of articles, respectively. Black/African American children were enrolled proportionally more than their U.S. population (odds ratio [OR] 1.88, 95% CI 1.87-1.89). Hispanic/Latino children were enrolled commensurate with their population (OR 1.02, 95% CI 1.01-1.03). American Indian/Alaska Native (OR 0.82, 95% CI 0.79-0.85), Asian (OR 0.56, 95% CI 0.55-0.57), and Native American/Pacific Islander (OR 0.66, 95% CI 0.61-0.72) children were enrolled significantly less compared to their population. White children were enrolled less than expected (OR 0.84, 95% CI 0.84-0.85), though represented 46.0% of participants in trials reporting race/ethnicity. Pediatric clinical trials conducted exclusively in Arizona, Tennessee, Georgia, West Virginia, and New Hampshire had the lowest mean diversity indices and those conducted in California, Maine, Illinois, and North Carolina had the highest diversity indices (Figure 2).
A substantial number of trials in high impact journals did not report participant race/ethnicity, and the method of such assignment was not noted in the majority that did report. Disparities exist in pediatric clinical trial enrollment among American Indian/Native Alaskan, Asian, and Native Hawaiian/Pacific Islander children. The higher representation of Black/African American children compared to U.S. population suggests inclusive research practices that could be extended to other historically disenfranchised racial and ethnic groups.
Figure 1. Reporting of participant race or ethnicity in published pediatric clinical trials and trial diversity index, 2011-2020
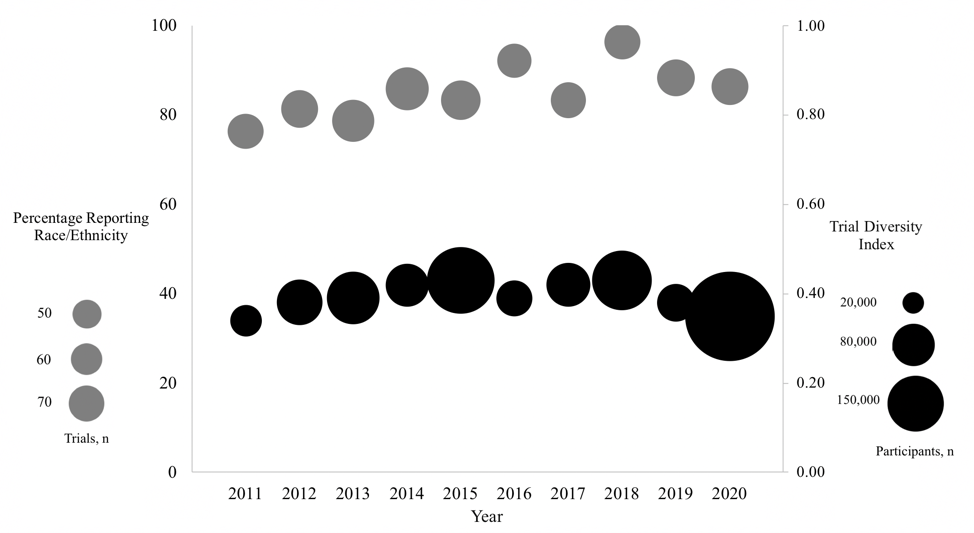
Figure 2. Pediatric clinical trial enrollment by state, among trials exclusively conducted in a single state.
exclusively conducted in a single state.