Last Updated
09/07/2022
Below are brief descriptions of the types of SARS-CoV-2 tests, when they should be used and how best to interpret their results.
SARS-CoV-2 Tests
Viral Tests: tests that detect the presence of SARS-CoV-2 nucleic acid or proteins. This category includes both NAATs and antigen tests.
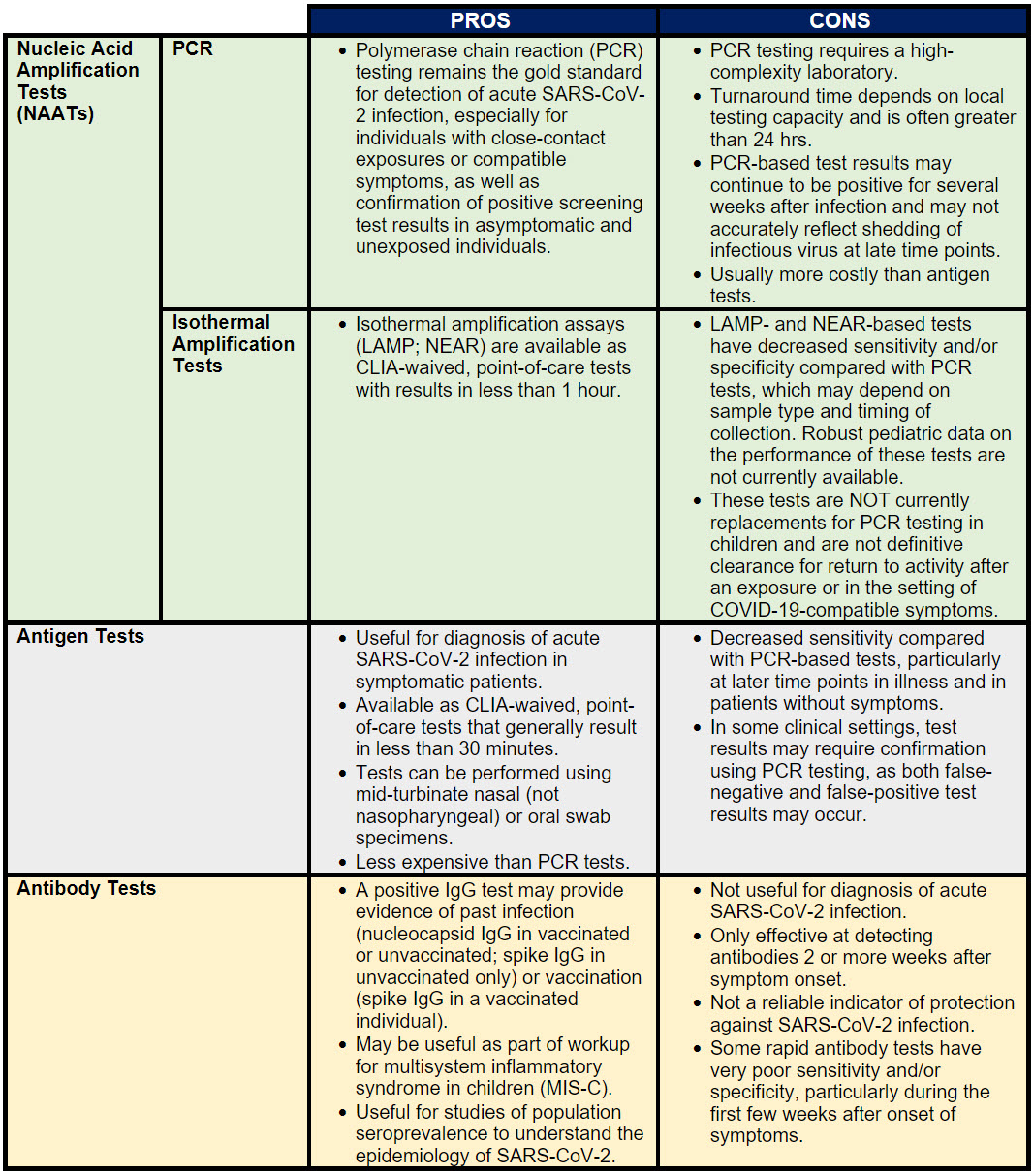
- Nucleic Acid Amplification Tests (NAATs) include polymerase chain reaction (PCR) tests as well as strategies for isothermal amplification of nucleic acid, such as loop-mediated isothermal amplification (LAMP) and nicking enzyme amplification reaction (NEAR). NAATs are generally performed on respiratory tract samples, most often nasal or nasopharyngeal swab specimens, and more recently, saliva samples. A PCR test that has received FDA authorization or approval is the “gold standard” for testing an individual child for acute SARS-CoV-2 infection.
- Antigen Tests are generally performed on nasal or oral swab specimens. Antigen tests generally have lower sensitivity than molecular tests, particularly in later stages of COVID-19. Some antigen tests have rapid turnaround at the point of care, making them useful for specific situations, including screening testing and at-home use, particularly for those who are symptomatic.
Nonviral Tests: tests that detect immune responses to SARS-CoV-2 infection or vaccination. This category includes antibody (serologic) tests.
- Antibody (Serologic) Tests can provide evidence of previous infection with SARS-CoV-2 but are not useful for the diagnosis of acute infection. A positive antibody test result does not prove that a patient has protection against SARS-CoV-2, and results of these tests should not be used to guide decisions about the need for vaccination or to group people in classrooms or other facilities. Individuals with positive antibody tests should continue to adhere to guidelines about masking, physical distancing and other preventive measures.
Test Selection
- Decisions about testing platforms may take into account local epidemiology and test characteristics such as sensitivity, specificity and positive/negative predictive values.
- Practical considerations such as test availability, turnaround time and cost may be important factors as well.
- There are positive and negative aspects to all available SARS-CoV-2 testing platforms. Some of these are reviewed in Table 1 below:
Table 1. Positive and Negative Aspects of SARS-CoV-2 Testing Platforms
Test Interpretation
Key Definitions
Sensitivity: Ability of the test to correctly identify those with SARS-CoV-2 infection (true positive rate).
Specificity: Ability of the test to correctly identify those without SARS-CoV-2 infection (true negative rate).
Pretest Probability: Likelihood of a particular individual patient having SARS-CoV-2 infection based on community prevalence, exposure, symptoms and other factors.
Positive Predictive Value (PPV): Probability that people who test positive are truly positive (ie, they have SARS-CoV-2 infection).
Negative Predictive Value (NPV): Probability that people who test negative are truly negative (ie, they do not have SARS-CoV-2 infection).
- Interpretation of SARS-CoV-2 test results depends on the sensitivity and specificity of the chosen test as well as community prevalence and the reason the test was performed (symptomatic vs exposure vs epidemiologic studies).
- Sensitivity and specificity are test characteristics that do not vary based on community prevalence. Available data for specific testing platforms may vary and pediatric data may not be available, particularly for newer tests made available under emergency use authorization.
- Sensitivity and specificity alone are not enough to determine a test’s reliability. Pretest probability must be combined with sensitivity and specificity to give the positive predictive value (PPV) and negative predictive value (NPV) of the test.
- Note that the context in which the test was performed, the result of a test, and the clinician and family’s risk tolerance in a given situation determines whether a particular test’s result is “good enough.”
- Receipt of SARS-CoV-2 vaccine does not result in positive NAAT or antigen test results. Antibody (serology) tests that detect responses to the spike (not nucleocapsid) protein may become positive following vaccination. Antibody testing is not recommended to determine immunity to COVID-19 following vaccination or to assess the need for vaccination.
- Interpretation of point-of-care or at-home testing performed by schools, workplaces, parents or others. There are several home tests with Emergency Use Authorization, with both molecular and antigen options available. These tests have become less costly and more readily available as schools, employers and public gatherings search for more convenient ways to exclude SARS-CoV-2 infection for asymptomatic individuals or those with mild/vague symptoms. Pediatricians are challenged to evaluate these test results because of the inability to verify the adequacy of the sample collection or whether the testing procedure was performed correctly. Generally, because of the high specificity of these tests, any positive result should result in home isolation. As these tests become more affordable and readily available, the absolute number of false-positive results will increase even if the relative rate of false-positive results remains very low. If the pretest probability is high (ie, high prevalence of disease in community, close-contact exposure, or symptoms), a NAAT (PCR) confirmation is generally not necessary after a positive at-home test result, and the patient should quarantine per routine and notify other potential contacts. If the pretest probability is low (ie, low community prevalence, no symptoms or exposures), a NAAT such as PCR should be considered. Specific situations are discussed in the COVID Testing Guidance.
Interim Guidance Disclaimer: The COVID-19 clinical interim guidance provided here has been updated based on current evidence and information available at the time of publishing. Additional evidence may be available beyond the date of publishing.
Last Updated
09/07/2022
Source
American Academy of Pediatrics