Angela S. Czaja1; Alexander Fiks 2,4; Christoph Hornik 3; Chad Livingston3; Lindsay Berrigan2; Lihai Song2; Jennifer Steffes4, Warren Bilker5, Robert Grundmeier2 on behalf of the Comparative Effectiveness Research through Collaborative Electronic Reporting (CER2) and Best Pharmaceuticals for Children Act – Pediatric Trials Network
1Dept Pediatrics, Critical Care, Univ of Colorado School of Medicine; Denver, CO 2Dept of Pediatrics, Perelman School of Medicine at the Univ of Pennsylvania and Children’s Hospital of Philadelphia; Philadelphia PA 3Duke Clinical Research Institute, Duke Univ, Durham NC; 4 Pediatric Research in Office Settings, The American Academy of Pediatrics, Itasca IL; 5Dept Biostatistics & Epidemiology, Perelman School of Medicine at the Univ of Pennsylvania, Philadelphia PA.
Presented at the 2018 Pediatric Academic Societies Annual Meeting.
Background: National guidelines recommend screening for metabolic syndrome after initiation of second- generation antipsychotic medications (SGAM), but guideline adherence may be poor.
Objective: Describe screening and detection rates of metabolic syndrome and weight changes among children & adolescents exposed to SGAM within a multi-center research network consisting of 222 U.S. pediatric primary care practices.
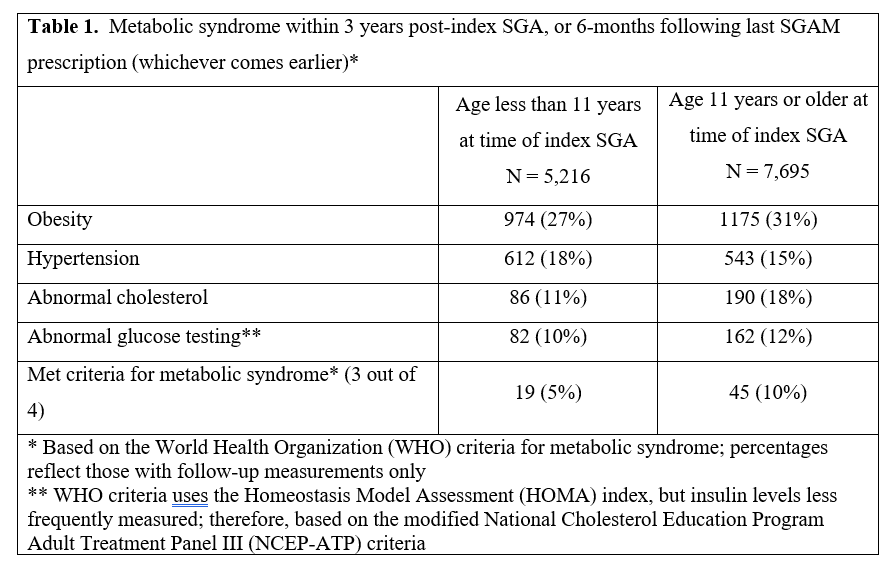
Design/Methods: We analyzed health data for 873,840 children aged 3–18 y with a minimum 180 days observation time from 2000-2016 to identify children prescribed SGAM. Measures of metabolic syndrome by World Health Organization definitions of obesity, hypertension, dyslipidemia, and high glucose were reported at baseline and after SGAM initiation (follow-up extended to 6-months after last SGAM prescription).
Changes from baseline weight measured at 3, 6, 12 mo for the exposed subjects were compared to propensity- matched unexposed subjects. Separate analyses were performed by age (<11y vs ≥11y) and sex.
Results: Over the study period, 12,911 (1.5%) eligible subjects had ≥1 SGAM prescription (50% risperidone, 30% aripiprazole, 14% quetiapine). Median treatment duration among children with ≥2 SGAM prescriptions was 1.1yr (IQR 0.4-2.3 yr). Males had younger age at SGAM initiation than females (47% vs 29% <11yrs).
After SGAM initiation, 56% had ≥1 BMI, 54% blood pressure measured, 14% had cholesterol testing and 16% had glucose testing. Only 851 (7%) had all four domains assessed, with obese children (BMI≥95%ile) more frequently tested (360/2145 [17%], p<0.001). Within the follow-up period, 64 children (7.5% of the 851 tested children) met criteria for metabolic syndrome (Table 1). Baseline weight was available for 4,986 (39%) exposed subjects. In all age/sex groups, the average weight gain during follow-up was greater for SGA-exposed than matched unexposed children, with greatest difference among males ≥11yrs (Table 2).
Conclusion(s): Screening for metabolic syndrome after SGAM initiation is infrequent in pediatric primary care, which is concerning given the accelerated weight gain observed. Prospective studies are needed to better delineate metabolic changes and identify targets for invention including greater adherence to screening recommendations.
Last Updated
10/15/2021
Source
American Academy of Pediatrics